- Home
- Student Information Center
Orientation Program In
Drug Regulatory Affairs & Pharmacovigilance
DURATION
- Day 1 : 1.5 hours per lecture. Total of lectures spread over morning and afternoon sessions.
- Day 2 : 1.5 hours per lecture. Total of 2 lectures in the morning session.
3 hours of interactive counselling and placement talk.
TRAINERS
- Professional trainers from Industry
- Subject Matter expert in Drug Regulatory Affairs (DRA) and pharmacovigilance (PV)
ELIGIBILITY
- B.Pharma 3rd & Final year students
- M.Pharma Students
- Bsc/Msc Microbiology, Biochemistry
- BDS & BAMS
REGISTRATION FEES
- Registration Fees : Rs. 2000/- Two days program.
- A booklet covering important points of DRA & PV of worth Rs. 470/-
- Certificate of Participation.
- Registration Fees : Rs. 1000/- One day program.
- Certificate of Participation.
How this Industrial Intership program help students in their career?
Why are we here?
- To improve the career prospects of the students in Drug Regulatory Affairs and Pharmacovigilance domains.
- To make the students industry ready so that they are not taken by the corporates as freshers but as trained professionals
- To give insight to the students on real time project.
- To make students aware of the demand of professionals in the pharma industry.
Specialized Course
- Integrated program in practical aspects of Drug Regulatory Affairs and Pharmacovigilance covering most in demand topics.
- Awareness of industry software such as ARIS g. Argus, Clinevo, etc.
- We shall also provide specialized session on skill and personality development.
What will we deliver?
- Make the students industry ready so that those are taken by corporates as experienced professionals and not as freshers.
- Opportunity to reach out to more than 500 pharma companies.
- The course is designed to learn DRA and PV as applied science.
- Overall personality development.
What goals can you achieve?
- Fulfilling global and domestic need of highly skilled professionals in DRA and Pharmacovigilance domain.
- Good scope of a promising job in the management cadre of top corporates.
- Good avenue for growth leading to an accelerated career growth.
In the programme, the students will get to know about the following in DRA & PV
- Overview of DRA and Pharmacovigilance
- Pharmacovigilance & DRA Programme in India including governing authorities across the globe.
- DRA & Pharmacovigilance functions, duties and their responsibilities in the industry.
- WHO & DRA
- ICH guidelines
- Summary of different licenses approval procedure
- Common technical documents used in RA
- Overview of Clinical Trials
- Individual Case Safety Reports
- Case Follow Up & Case Study Report
- Skill required for Drug Regulatory & Drug Safety Positions
- What are the career opportunities in DRA & Pharmacovigilance
- Valuable skills you’ll Learn in a DRA & Pharmacovigilance Programme
- Pharmacovigilance safety expert skills for your resume and career
- Hands-on-training on various DRA & PV softwares (Sugam, Clinevo Drug Safety etc.), NDA; IND and ANDA applications etc.
- Regulatory Affairs and Pharmacovigilance are the two important pillars of Pharma Industry and are the focussed points of learning in these programme.
BENEFITS
This programme helps the students in understanding and gaining in-depth knowledge & hands on training in the domains of Drug Regulatory Affairs & Pharmacovigilance as per industry standards.
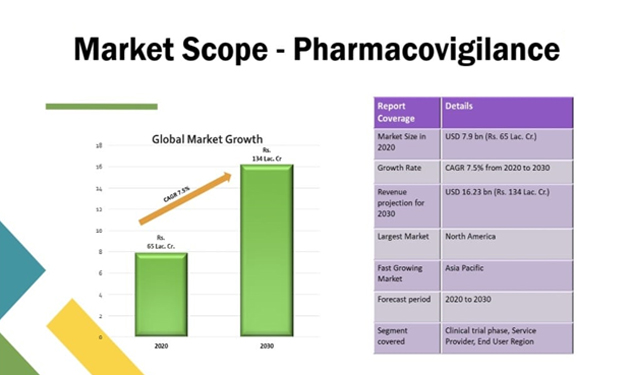
Market Scope
Go with the highest booming departments like Pharmacovigilance (PV) & Drug Regulatory Affairs (DRA). Now future will be in DRA & PV. Make it with PRI